Drug development business
We are developing innovative drugs for severe asthma
“We would be grateful if you could be a business partner and support our drug development”
→ Indications:Steroid-resistant Severe Asthma
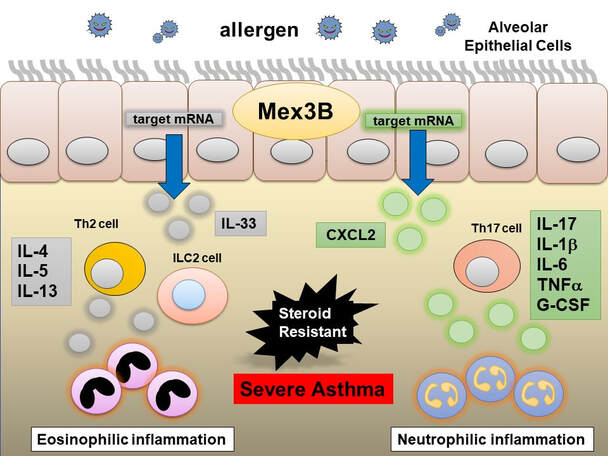
→ An antisense oligonucleotide targeting the mRNA of Mex3B
→ Mex3B is an RNA binding protein that induces cytokines involved in airway inflammation
→ Route of administration: inhalation
→ status: clinical trial (First in Human)
→ An antisense oligonucleotide targeting the mRNA of Mex3B
→ Mex3B is an RNA binding protein that induces cytokines involved in airway inflammation
→ Route of administration: inhalation
→ status: clinical trial (First in Human)
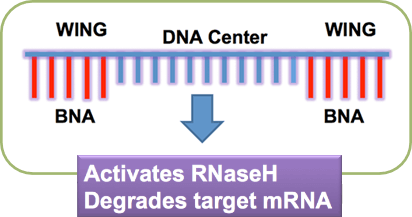
Structure of Gapmer-type
antisense oligonucleotide
antisense oligonucleotide
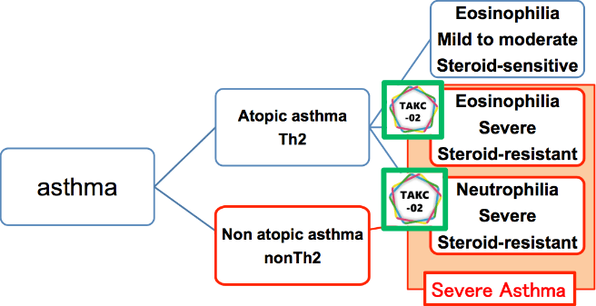
Fig 1. Clinical Significance of TAKC-02
→ the first drug for neutrophilic severe asthma
→ effective for both neutrophilic and eosinophilic severe asthma
Ref:
The RNA Binding Protein Mex-3B Is Required for IL-33 Induction in the Development of Allergic Airway Inflammation.
Yamazumi et al Cell Rep. 2016 Aug 30;16(9):2456-71.
The RNA-binding protein Mex-3B plays critical roles in the development of steroid-resistant neutrophilic airway inflammation.
Yamazumi et al Biochem Biophys Res Commun. 2019 Nov 5;519(2):220-226.
TAKC-02 is a drug developed by TAK-Circulator based on the results of joint research with the Institute for Quantitative Biosciences (IQB) the University of Tokyo (Project Professor Tetsu Akiyama).
→ effective for both neutrophilic and eosinophilic severe asthma
Ref:
The RNA Binding Protein Mex-3B Is Required for IL-33 Induction in the Development of Allergic Airway Inflammation.
Yamazumi et al Cell Rep. 2016 Aug 30;16(9):2456-71.
The RNA-binding protein Mex-3B plays critical roles in the development of steroid-resistant neutrophilic airway inflammation.
Yamazumi et al Biochem Biophys Res Commun. 2019 Nov 5;519(2):220-226.
TAKC-02 is a drug developed by TAK-Circulator based on the results of joint research with the Institute for Quantitative Biosciences (IQB) the University of Tokyo (Project Professor Tetsu Akiyama).
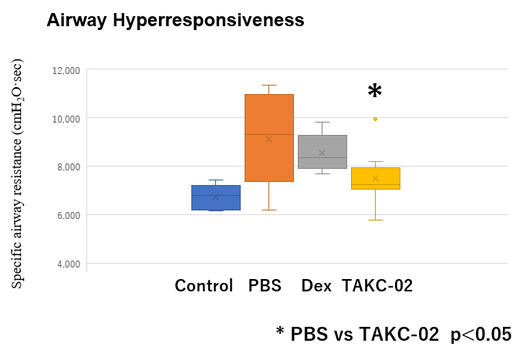
Fig 2. TAKC-02 Suppresses Airway Hyperresponsiveness in the mouse model
WT mice were sensitized with OVA/Alum (for eosinophilic airway inflammation) or OVA/CFA (for neutrophilic airway inflammation) and nebulized with OVA (solution) or PBS from day 21-23 and analyzed on day 24. Aerosolized Antisense Oligonucleotides was administered for 5 days.
WT mice were sensitized with OVA/Alum (for eosinophilic airway inflammation) or OVA/CFA (for neutrophilic airway inflammation) and nebulized with OVA (solution) or PBS from day 21-23 and analyzed on day 24. Aerosolized Antisense Oligonucleotides was administered for 5 days.
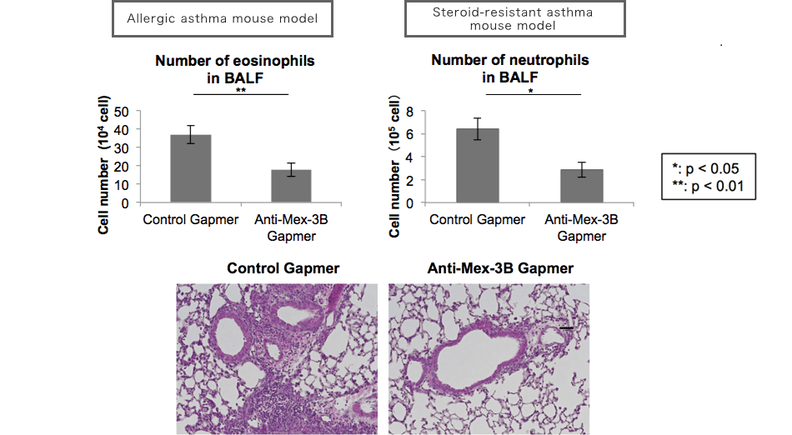
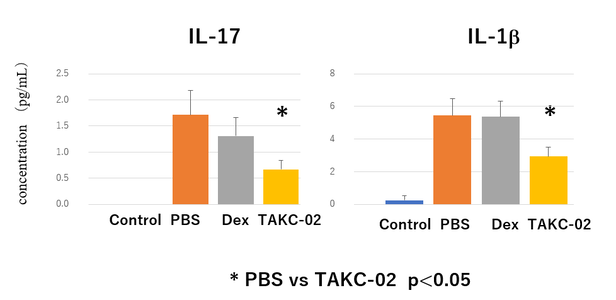
Fig 3. The effect of TAKC-02 for Airway Inflammation model
Inhalation of anti-Mex-3B Antisense Oligonucleotides suppresses neutrophilic steroid-resistant airway inflammation (Dexamethasone was injected to examine the model is steroid-resistant). WT mice were OVA/CFA on days 0, nebulized with OVA (solution) or PBS from day 21-23 and analyzed on day 24. Aerosolized Antisense Oligo was administered 5 times. We could nicely obtain sufficient pharmacological evidence of TAKC-02 in pre-clinical studies which were conducted in a contract research organization (CRO).
TAKC-02 Suppresses Airway Hyperresponsiveness.
Inhalation of anti-Mex-3B Antisense Oligonucleotides suppresses neutrophilic steroid-resistant airway inflammation (Dexamethasone was injected to examine the model is steroid-resistant). WT mice were OVA/CFA on days 0, nebulized with OVA (solution) or PBS from day 21-23 and analyzed on day 24. Aerosolized Antisense Oligo was administered 5 times. We could nicely obtain sufficient pharmacological evidence of TAKC-02 in pre-clinical studies which were conducted in a contract research organization (CRO).
TAKC-02 Suppresses Airway Hyperresponsiveness.
TAKC-02 Suppresses IL-17 and IL-1β(measured in BALF)
Fig 4. TAKC-02 Suppresses both Eosinophilic and Neutrophilic Inflammation.